Free Case Evaluation
You will never be charged a fee unless a recovery is made for you.
We are no longer accepting new cases.
Exactech knee and ankle lawsuits are being filed by people who received one of the since-recalled Exactech knee or ankle implants and experienced complications requiring revision surgery. Patients who need revision surgery may be experiencing one or more of these complications:
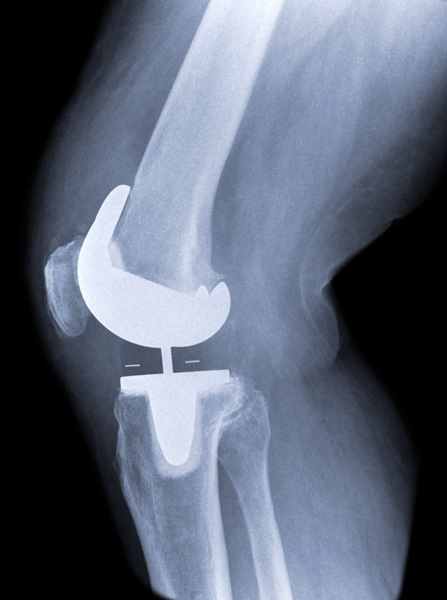
Trustwell Law is accepting cases from patients experiencing complications from Exactech knee replacements implanted between 2004 and February 2022 provided they need revision surgery or had revision surgery within 12 years of the original implant. We are not accepting cases for individuals who have had their implant for 12 years or longer without revision.
Trustwell Law is also accepting Exactech ankle lawsuits from patients implanted with a recalled Exactech ankle between 2017 and February 2022.
If you or a loved one suffered severe complications following an Exactech knee or ankle replacement surgery, call us at 800-796-1636 or submit your case details online, and someone will contact you shortly. You pay nothing unless your lawsuit is successful and you receive compensation.
At Trustwell Law, our experienced attorneys take a personalized, compassionate approach. We cut through the legalese and partner with our clients. We have access to the expertise, resources, and manpower to fully investigate each case and fight for and with our clients to get the justice they deserve.
The models and parts involved in the Exactech knee and ankle implant recall are:
About 147,732 of the recalled devices have been implanted in the U.S. since 2004.
Exactech is recalling the knee and ankle implants because the polyethylene plastic inserts used in the implants may wear down too soon, leading to implant failure and the need for revision surgery. The recalled Exactech Ultra-High Molecular Weight Polyethylene (UHMWPE) Knee and Ankle Polyethylene Inserts fit between metal components in knee and ankle replacements to cushion joints.
Beginning in 2004, these implants were not packaged to specification because the devices came in vacuum bags missing a secondary barrier layer. That secondary barrier layer, when present, contains ethylene vinyl alcohol to enhance the first layer’s oxygen resistance. The not-to-specification packaging does not adequately protect the inserts from oxygen exposure, which can cause the inserts to degrade and deteriorate over time and lead to device failure. In the recall letter to surgeons, the company acknowledged that the “Optetrak Knee system has shown statistically significant higher overall revision rates as compared to other TKA’s in the Australian, United Kingdom and New Zealand registries.”
The letter to surgeons also says that “surgeons should maintain an appropriate index of suspicion for patients with any new or worsening pain, inability to bear weight, grinding or other noise, swelling, or instability in their knee or ankle.”
Exactech’s recalled knee and ankle implants may be subject to:
Patients with the recalled implants may suffer from:
These issues and symptoms may require revision surgery to correct.
If you or a loved one received an Exactech knee or ankle implant and wish to consult an attorney, contact us for a free consultation. You may be entitled to compensation for medical bills, pain and suffering, lost wages, and other expenses.
Sources
Encyclopedia of Surgery. (n.d.) Knee revision surgery. Retrieved from https://www.surgeryencyclopedia.com/Fi-La/Knee-Revision-Surgery.html#b
Exactech. (2022, February 7). Exactech Ankle Patient Letter. Retrieved from https://www.exac.com/product/exactech-ankle-patient-letter/
Exactech. (2022, February 7). Exactech DHCP Letter. Retrieved from https://www.exac.com/product/exactech-dhcp-letter/
Exactech. (2022, February 7). Exactech Knee Patient Letter. Retrieved from https://www.exac.com/product/exactech-knee-patient-letter/
Exactech. (nd). US Exactech Recall Information, Knee/Ankle. Retrieved from https://www.exac.com/medical-professionals/recall-information/
Mayo Clinic. (2017, December 29). Knee replacement. Retrieved from https://www.mayoclinic.org/tests-procedures/knee-replacement/about/pac-20385276
Medline Plus. (2019, September 11). Knee Replacement. Retrieved from https://medlineplus.gov/kneereplacement.html
You will never be charged a fee unless a recovery is made for you.