Free Case Evaluation
You will never be charged a fee unless a recovery is made for you.
We are no longer accepting new cases.
Trustwell Law is accepting cases on behalf of people who took Elmiron to treat interstitial cystitis (IC) and developed vision problems and experienced damage to their retinas.
If you are considering filing an Elmiron lawsuit, call us at 800-796-1636 or submit your case details online and someone will contact you shortly. You pay nothing unless your lawsuit is successful and you receive compensation.
At Trustwell Law, our experienced attorneys take a personalized, compassionate approach. We cut through the legalese and partner with our clients. We also have access to the expertise, resources, and manpower to fully investigate each case and fight for and with our clients to get the justice they deserve.
Dr. Nieraj Jain at the Emory Eye Center in Atlanta, Georgia first suspected that prolonged use of Elmiron to treat interstitial cystitis, also called painful bladder syndrome, could be damaging the retinas of some patients. Using advanced retinal imaging, he found that six Emory Eye Center patients, all women who had been taking Elmiron for IC, had eye damage doctors had never seen before. Calling these symptoms “retinal maculopathy,” the doctors found the pigment cells within these women’s retinas literally had changed color.
Ophthalmologists in northern California then began to review the histories and health of their patients who had been taking Elmiron. They found that about 25 percent of patients with significant exposure to Elmiron showed signs of eye damage.
Elmiron is one of only two drugs approved by the FDA to treat interstitial cystitis.
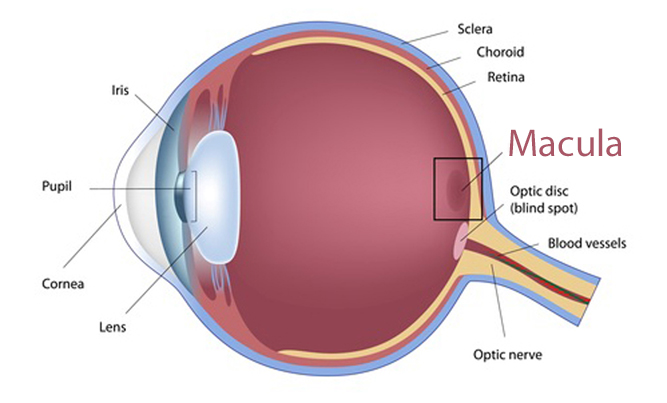
The retina is a thin layer of tissue located at the back of the eye. It contains millions of tiny light-sensing nerve cells commonly called “rods” and “cones” because of their shapes.
Cones are found at the center of the retina in an area called the macula. Cones provide sharp clear vision in bright light, detecting colors and details. Rods are located outside the macula and extend to the edge of the retina. Rods handle peripheral (side) vision; they also detect motion and help us see in dim light.
When examining Elmiron patients with eye damage, doctors have found changes in the maculae. Vision changes and complaints reported by these patients include:
Before the link to Elmiron was discovered, these vision problems and eye injuries may have been misdiagnosed as:
However, physicians and researchers have now identified a unique and discernable pattern of injuries in Elmiron patients with eye damage and named it “pigmentary maculopathy.”
Doctors have found that, when caught early, the pigmentary maculopathy damage may be mitigated by stopping the medication. [Warning: Never stop taking a prescribed medication without consulting your physician.] However, if the pigmentary maculopathy remains undiagnosed and the patient continues taking Elmiron, the injuries can lead to permanent vision loss.
Now that the medical community knows of the relationship between eye injuries/retinal damage and Elmiron (pentosan polysulfate sodium), doctors recommend patients taking Elmiron should receive eye exams at least annually.
Elmiron, or Pentosan Polysulfate Sodium (PPS)
Elmiron is one of only two drugs the FDA has approved to treat IC. Elmiron’s listed common side effects are:
Previously known, more serious but less likely, side effects of Elmiron include:
Anyone taking Elmiron who experiences any of these more serious side effects should immediately report them to their doctor.
The FDA has now added eye disorders to its list of Potential Signals of Serious Risks for Elmiron (PPS) and is reportedly evaluating the need for regulatory action concerning Elmiron and eye injuries and vision damage.
If you or a loved one took Elmiron to treat interstitial cystitis (IC) and developed vision problems and experienced damage to their retinas, contact us for a free consultation.
Sources
American Academy of Ophthalmology. (2019, October 12). More evidence linking common bladder medication to a vision-threatening eye condition. Retrieved from https://www.sciencedaily.com/releases/2019/10/191012141218.htm
American Optometric Association. (n.d.). How Your Eyes Work. Retrieved from https://www.aoa.org/patients-and-public/resources-for-teachers/how-your-eyes-work
Cunha, J. (2019, May 31). Elmiron. Retrieved from https://www.rxlist.com/elmiron-side-effects-drug-center.htm
Ferguson, T., et al. (2019, January 5). Chronic use of pentosan polysulfate sodium associated with risk of vision-threatening disease. Retrieved from https://link.springer.com/content/pdf/10.1007/s00192-018-3850-9.pdf
Hanif, A., and N. Jain. (2019, July 10). Clinical Pearls for a New Condition. Retrieved from https://www.reviewofophthalmology.com/article/clinical-pearls-for-a-new-condition#:~:text=A%20unique%20pigmentary%20maculopathy%20is,best%20appreciated%20by%20using%20FAF.
Hanif, A., et al. (2019, September 5). Phenotypic Spectrum of Pentosan Polysulfate Sodium–Associated Maculopathy A Multicenter Study. Retrieved from https://jamanetwork.com/journals/jamaophthalmology/article-abstract/2749093
Hicks, Tony. (2019, October 14). This Common Bladder Medication May Damage Your Vision. Retrieved from https://www.healthline.com/health-news/common-bladder-medication-may-damage-vision#:~:text=Researchers%20say%20the%20bladder%20medication,have%20eye%20exams%20every%20year
Pearce, W., R. Chen, and N. Jain. (2018, May 22). Pigmentary Maculopathy Associated With Chronic Exposure to Pentosan Polysulfate Sodium. Retrieved from https://pubmed.ncbi.nlm.nih.gov/29801663/
U.S. Food and Drug Administration. (n.d.). July – September 2019 | Potential Signals of Serious Risks/New Safety Information Identified by the FDA Adverse Event Reporting System (FAERS). Retrieved from https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/july-september-2019-potential-signals-serious-risksnew-safety-information-identified-fda-adverse
You will never be charged a fee unless a recovery is made for you.