Free Case Evaluation
You will never be charged a fee unless a recovery is made for you.

We are no longer accepting new cases.
Trustwell Law is accepting cases on behalf of persons born with certain congenital abnormalities (birth defects) affecting the face, heart, genitals, or head that have been linked to maternal use of the prescription drugs Provigil and Nuvigil during pregnancy.
If you or your child were born with birth defects and are considering filing a Provigil or Nuvigil lawsuit, call us at 800-796-1636 or submit your case details online. A member of our legal team will contact you shortly. You may be entitled to compensation.
Our attorneys have years of experience and a reputation for personalized, compassionate partnering with our clients. We also have access to the expertise, resources, and manpower to fully investigate your circumstances and get you the justice you deserve.

Provigil (modafinil) and Nuvigil (armodafinil) were approved by the U.S. Food and Drug Administration (FDA) to improve wakefulness in adults suffering from excessive sleepiness due to:
Provigil and Nuvigil have been popular and very profitable for years but recently made news for other reasons.
In June 2019, Teva Pharmaceuticals issued public notices to health-care providers in Europe and Canada warning that modafinil use during pregnancy “is suspected to cause congenital malformations [birth defects].” To date, there have been no such warnings issued in the United States. The European and Canadian warnings seem to apply to both Provigil and Nuvigil because they have the same chemical formula. Parents of children born with birth defects whose mothers used either drug while pregnant have begun to file lawsuits.
The birth defects associated with exposure to modafinil in the womb affect the face, heart, genitals, and head, including:
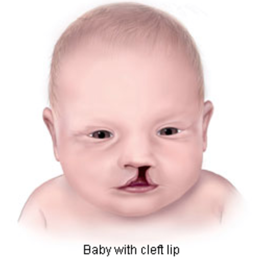
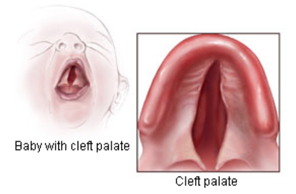
Image Credit: Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities
According to the warning letter—issued by Teva Ireland in cooperation with the European Medicines Agency and Ireland’s Health Products Regulatory Authority—Teva’s data show a rate of major birth defects associated with maternal use of modafinil approaching 15 percent. By comparison, the rate of congenital defects in the general population is roughly 3 percent. So the data suggests babies whose mothers take modafinil while pregnant are five times more likely to have a birth defect than babies whose mothers don’t take the drug
Also in June 2019, based on Teva Canada’s report of the same data, Health Canada issued its own safety alert for modafinil. Health Canada is the Canadian government agency responsible for public health. The alert stated that Teva’s data “suggested a higher rate of major congenital anomalies, and other adverse reactions” in children exposed to modafinil in the womb and warned that the drug is contraindicated in women who are or may become pregnant.
The reported data on birth defects came from the 2018 annual report of the Nuvigil/Provigil Pregnancy Registry. The FDA asked the manufacturer to establish the registry to gather data on pregnancies and fetal outcomes associated with Provigil and Nuvigil in the United States, because testing during the approval process had shown some evidence of developmental harm in animals.
Provigil and Nuvigil stimulate the central nervous system. By doing so, they reduce the excessive sleepiness resulting from narcolepsy, OSA, or SWD, without treating the underlying medical condition.
As non-amphetamine-based stimulants, Provigil and Nuvigil have also been widely prescribed for “off-label” uses, including treating symptoms of Attention Deficit Hyperactive Disorder (ADHD) and several mood-related psychiatric conditions. Provigil became a blockbuster drug in the early 2000s, with yearly U.S. sales exceeding $1 billion before generic versions entered the market. Many of those sales were for unapproved uses.
By 2012, off-label use of Provigil had become so prevalent that the American Academy of Sleep Medicine (AASM) issued a statement warning its members and the public against off-label use. According to the AASM, “Provigil should not be prescribed off-label or purchased independently by consumers for cognitive or performance enhancement. There is little evidence to support the use of Provigil or any other drug to improve learning and memory, and no medication provides such benefits without side effects.”
Doctors can legally use their medical judgment to prescribe any drug for any purpose, but federal law mandates that drug companies may only promote their drugs for approved uses for which the FDA has found them safe and effective.
Authorities claimed Provigil’s manufacturer, Cephalon, Inc., violated those laws, leading to years of legal battles. As Provigil sales grew rapidly after its 1998 approval, Cephalon faced allegations of improper marketing followed by criminal and civil lawsuits. In these cases, the federal government, many state governments, and private whistleblowers claimed the company systematically promoted several drugs (including Provigil) for off-label uses. In 2008, Cephalon pled guilty to one criminal count based on its illegal marketing and paid $50 million in criminal fines and asset forfeitures. Cephalon also agreed to pay $375 million plus interest to settle related civil lawsuits under the False Claims Act.
Even so, when Teva Pharmaceuticals bought Cephalon in 2011 it inherited a massive round of antitrust lawsuits based on Cephalon’s alleged past practice of paying other drug companies not to sell generic versions of Provigil. In 2008, the Federal Trade Commission claimed Cephalon had violated antitrust laws by paying generic drug manufacturers more than $300 million to stay off the market until 2012 as part of settling patent infringement cases. Many similar civil lawsuits followed, filed by pharmacies, insurers, and drug wholesalers. By 2015, Teva agreed to pay a total of $1.2 billion to settle those antitrust claims.
Health plans and other indirect purchasers of Provigil filed class action lawsuits against Teva and Cephalon based on the allegations in the antitrust cases, which Teva settled in early 2019 for a payment of almost $66 million.
There have been no outcomes announced (verdicts, settlements, dismissals) in any Provigil or Nuvigil lawsuit based on birth defects.
End Notes
Article Sources
You will never be charged a fee unless a recovery is made for you.